Are you trying to figure out the best way to do a 1 to 10 dilution? Don’t worry, we’ve got your back! In this blog, we’ll break down the process step-by-step so that you can easily complete a 1 to 10 dilution with confidence. So grab your lab coat and come join us in the world of science!
What is a dilution of 1 to 10?
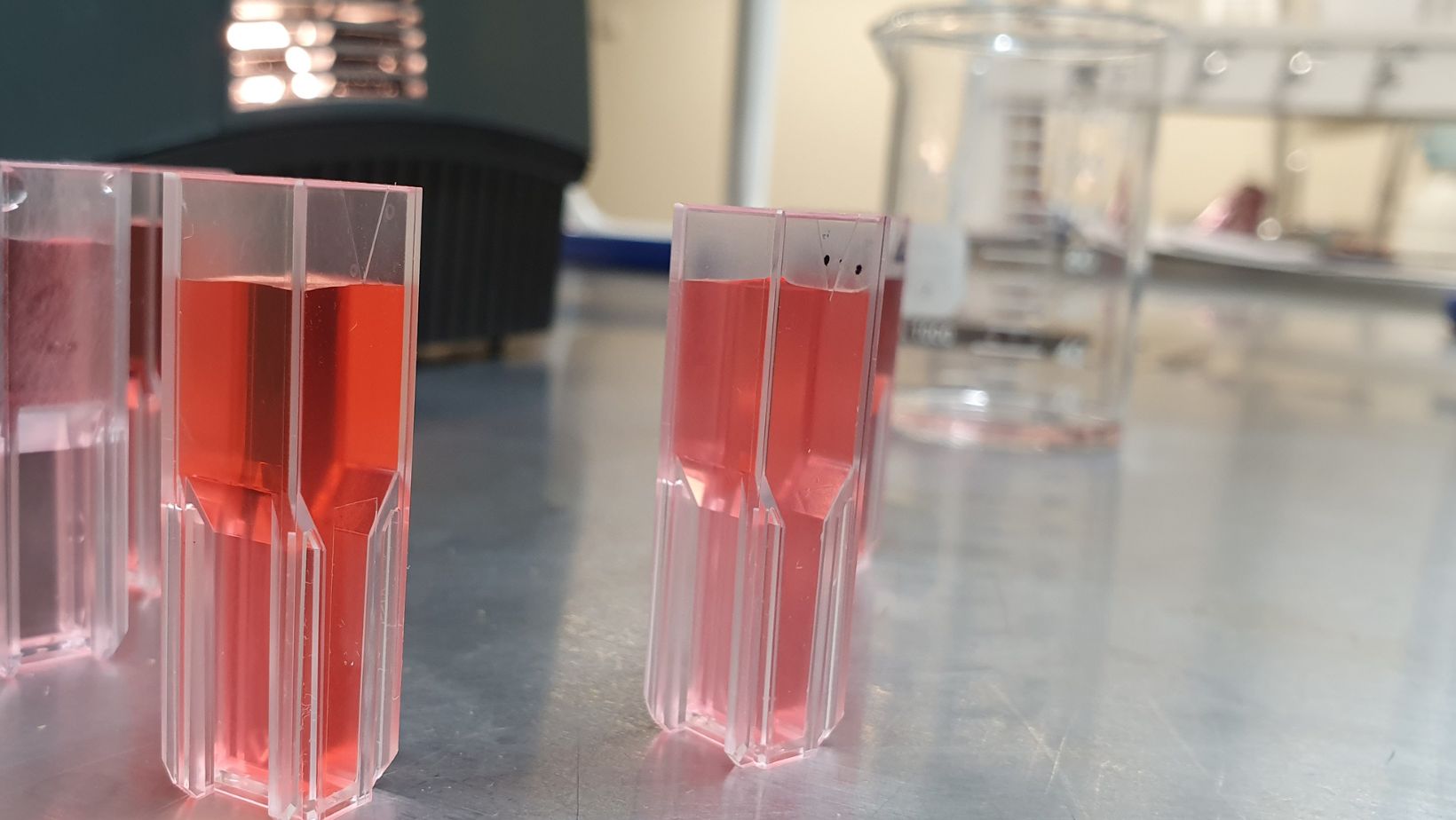
A dilution of 1 to 10 is one of the most common dilutions used in laboratory studies. It refers to a solution or sample that has been diluted to one-tenth of its original concentration. To make this dilution, you will need two vessels (beakers, flasks, etc).
First, take the original sample material and add it to the first vessel. Measure out an appropriate volume starting with the amount of material in the first vessel and then another equal volume from a stock solution, distilled water or other suitable solvent (this may depend on what you are working with, e.g., a chemical vs a type of bacteria).
Mix together thoroughly – this is now your 1:10 dilution now that the original material has been reduced by 10x. You can easily make even more dilute solutions if needed by continuing to add an equal amount from your starting material to an equal amount of solvent until you reach your desired concentration level.
What does a one-to-one dilution factor imply?
A one-to-one dilution factor implies that the concentrations of solute in the original solution and the resulting solution are identical. To illustrate this, if a solution were to start with a 1 molar concentration and a 1 to 10 dilution were to be performed, that would imply that after the dilution was completed, the concentration in the final solution would be 0.1 molar. A one-to-one dilution results in no change of concentration since it implies an equal amount of solute and solvent for both starting and ending solutions.
What is serial dilution and how does it work?
Serial dilution is a procedure used to decrease the concentration of a solution by diluting it with liquid. This technique is often used in chemical and microbiological analysis to adjust the concentration of reactants or to quantify the concentration of certain chemical or microbial components. Serial dilution requires that successive volumes of sample and diluent be mixed together and volumetrically measured.
To perform a serial dilution, begin by estimating the initial concentration of your sample or preparing a stock solution. Take this stock solution, measure out a known volume, and add it to an equal volume of liquid for each subsequent step in the series. This process can be repeated 1-10 times depending on your results and desired concentrations. After each addition, carefully mix the contents so that they are homogenous before moving to the next step. Once finished, you will have multiple tubes containing various concentrations which can then be tested against appropriate media or reagents for analysis.
What is the significance of repeated dilution?
The practice of diluting a solution is an important tool in scientific methodology, allowing scientists to precisely adjust concentrations through the use of previously measured solutions. Repeated dilutions are done with increasing dilution ratios, typically ranging from 1:2 to 1:10, and they provide even more precision when measuring. For example, a sample could be diluted from its original concentration to four lower concentrations by repeating the original dilution step four times.
Repeated dilutions are especially important for laboratories that require extremely accurate measurements for their testing protocols. Because each step of the process results in further reductions in concentration, errors that occur during the initial solutions preparation are reduced over time, making it easier to obtain accurate results. In addition, repeat-dilutions can be used to determine how reactive different substances are when mixed together. This is accomplished by adding different substances into a series of solutions at varying concentrations and measuring any observed changes over time.
By relying on the inverse relationship between concentration and solubility, scientists can create repeatable test scenarios that take into account all variables before testing begins. As such, repeated dilutions enable laboratory professionals to consistently achieve precise solutions without sacrificing accuracy or reliability.